The topic of acid base imbalances (Respiratory and Metabolic Acidosis & Alkalosis) is an important topic, that a lot of nursing students struggle with. This post will break it down in an easy to understand way, while also helping you keep it to memory.
Key Points
Before you answer any questions on respiratory acidosis/ alkalosis and metabolic acidosis/alkalosis, you should understand the following.
The expected normal PH level is 7.35-7.45, with anything <7.35 being acidic and anything >7.45 being alkaline.
Carbondioxide (PCO2) is the key player in the respiratory segment, while hydrogen ions/bicarbonate (HCO3) are the key players in the metabolic segment.
Whenever we say “respiratory”, we are referring to the lungs, and whenever we say “metabolic”, we are referring to the kidneys/our whole metabolic system.
When we breathe in and out, we breathe in oxygen and breathe out carbondioxide; there is always a balance. Too much or too little carbondioxide is bad for the body.
In the same way, when the kidneys filter our blood every minute, they regulate the amount of hydrogen ions in our bodies; there is a balance. Too much hydrogen ions/bicarbonate or too little hydrogen ions/bicarbonate is bad for the body.
Lastly, bicarbonate (HCO3) is a base that helps to balance out the hydrogen ions in our bodies to prevent metabolic acidity.
Respiratory Acidosis/Alkalosis
Based on our key points above, we can say that respiratory refers to the lungs and acidosis refers to a PH <7.35. In the same way, alkalosis refers to the PH> 7.45 as it relates to the lungs.
Respiratory acidosis occurs when we do not exhale the appropriate amount of carbondioxide that we should, causing us to have too much in our body. This occurs because a person is breathing too slow or “hypoventilating (Respiratory rate may be <12 and oxygen saturation may be <90%)”
Respiratory alkalosis occurs when we exhale too much carbon-dioxide than we should, causing us to have too little in our body. This occurs because a person is breathing too fast or “hyperventilating (Respiratory rate may be >20 and oxygen saturation may be <90%)”
Metabolic Acidosis and Alkalosis
Based on our key points above, we can say that metabolic refers to the kidneys/metabolism and acidosis refers to a PH <7.35. In the same way, alkalosis refers to the PH> 7.45 as it relates to the kidneys.
Metabolic acidosis occurs when our kidneys cannot remove the appropriate amount of hydrogen ions that it should, causing us to have too much in our body.
Metabolic alkalosis occurs when our kidneys remove too much hydrogen ions than it should, causing us to have too little in our body. It can also occur if a patient has excessive vomiting,diarrhea etc and they lose hydrogen ions through that too.
Metabolic alkalosis can also occur when we have too much Bicarbonate (HCO3) in our body.
Diagnosis of These Conditions
The main test used in the hospital to determine respiratory and metabolic acidosis/alkalosis, is called the arterial blood gas (ABG). The main things this test will measure are the PH, PACO2 (partial pressure carbondioxide), and HCO3 (bicarbonate).
The results from these numbers can hep clue you into what the patient might have, just by knowing what you are looking at.
Normal PH as we discussed is 7.35-7.45, normal PACO2 is 35-45 (<35 is too little carbondioxide and >45 is too much carbondioxide).
Lastly, normal HCO3 is 21-28 (<21 is too little bicabonate and >28 is too much bicarbonate).
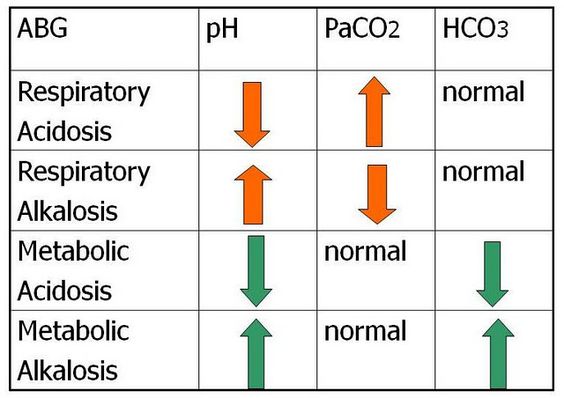
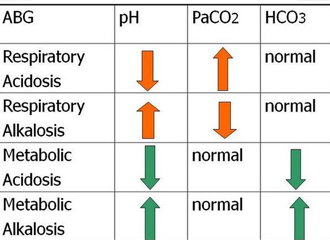
Let Us Examine The Picture Above
Use the mnemonic ROME (Respiratory opposite, Metabolic equal). With respiratory, the PH and carbondioxide (CO2) are usually going opposite directions. With metabolic, the PH and bicarbonate (HCO3) are usually going the same direction.
The first row is the respiratory acidosis, so it involves the PH and the carbondioxide (PaCO2). In respiratory acidosis, the PH is low (<7.35) because we have too much carbondioxide (PaCO2) in the body.
The second row is the respiratory alkalosis, so it involves the PH and the carbondioxide (PaCO2). In respiratory alkalosis, the PH is high (>7.35) because we have too little carbondioxide (PaCO2) in the body.
The third row is metabolic acidosis, so it involves the PH and the bicarbonate (HCO3). In metabolic acidosis, the PH is low (<7.35) because we have too much hydrogen ions (h+) in our body or too little bicarbonate (HCO3).
The last row is metabolic alkalosis, so it involves the PH and the bicarbonate (HCO3). In metabolic alkalosis, the PH is high (>7.35) because we have too little hydrogen ions (h+) or too much bicarbonate (HCO3)
Key Take Away
Respiratory refers to the lungs, and metabolic refers to the kidneys
Acidity means <7.35 (low) and alkalinity means >7.35 (high).
If PH is low (<7.35) and carbondioxide (PCO2) is high, think respiratory acidosis. If PH is high (>7.35) and carbondioxide is low, think respiratory alkalosis.
If PH is low (<7.35) and bicarbonate (HCO3) is high, think metabolic acidosis. If PH is high (>7.35) and bicarbonate is low, think metabolic alkalosis.

There you have it. Leave me a comment below if this helped you understand respiratory and metabolic acidosis/alkalosis, a little better. Don’t forget to check out some of my other posts especially these.